Even though we may believe we understand how water behaves, we have been ignoring what happens when it enters places that are only one molecule thick. In comparison to water in bulk, the results turn out to be considerably different.
When the solid, liquid, and gas phases of water come into contact, icebergs can form. However, there is a lot more going on that we cannot see, including multiple phases of matter that can only exist in extremely abrasive environments. As scientists discover new techniques to alter and measure matter, we typically find more of these.
The most recent instances happen when water is locked in an environment where it forms a sheet just one molecule thick, at which time many phases can coexist simultaneously. Not all of them need to be created under extreme pressure or energy, in contrast to several other phases of matter. The difficulty was instead in observing their behaviour and comprehending the structures, a topic that has recently been covered in a research that was published in Nature.
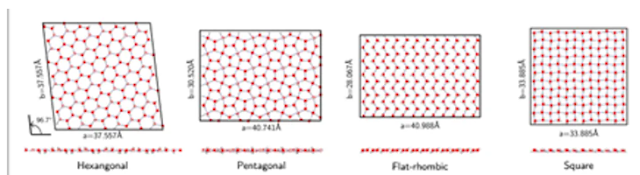
Dr. Venkat Kapil of the University of Cambridge and colleagues used computational modelling to discover that water forms a phase that resembles bulk ice at low pressure, with molecules organised in a hexagonal pattern and a melting point that is roughly 100° C lower than the three-dimensional version.
The molecules rearrange themselves into pentagonal and then rhombic shapes as pressure or temperature rises. The water enters what the authors refer to as the "hexatic" phase at pressures they refer to as "intermediate" (about 8,000 atmospheres). When temperatures rise above roughly 70°C (158°F), water behaves in a manner that is halfway between solid and liquid, with fixed but revolving molecules.
The water turns superionic when the pressure rises further. It is very electrically conductive but resembles ice more than water. However, neither protons nor electrons carry the current. Superionic phases can also exist in bulk water, however doing so requires much greater pressures.
It's not always the case that other phases of matter, like those that form at extremely low temperatures or under intense pressure, are more interesting to study than useful. All kinds of porous materials have tiny spaces, and whether or not we need membranes, single-molecule thick layers can form between them.
When there aren't enough room inside for bulk water, the authors claim our own bodies contain some of these phases, and their response may have an impact on how well medical treatments work. Water has also been a component of water desalination and battery projects for a long time, but we haven't known how it affects productivity.
Understanding how water behaves is the fundamental question in all of these fields, according to Kapil. "With unparalleled prediction precision, our method enables the analysis of a single layer of water in a graphene-like channel."
The authors note that the hexatic phase behaviour is mostly consistent with earlier hypotheses, while the superionic pushes us further into the unknown.
One way to think of this phase is that oxygen atoms form a solid lattice, and protons flow like a liquid through the lattice, like children running through a maze, said Kapil. "The existence of the superionic phase at easily accessible conditions is peculiar, as this phase is generally found in extreme conditions like the core of Uranus and Neptune," he said.
The authors want to enhance battery architecture by taking advantage of the superionic phase's excellent conductivity.
Reference:Nature




.jpg)
0 Comments